
Vol. 41, n.º 1, 2008
REVISTA
ESPAÑOLA DE
Vol. 41, n.º 1, 2008 |
REVISIONES
Brian Eyden
Department of Histopathology, Christie Hospital
NHS Trust, Manchester, United Kingdom.
Brian.Eyden@christie-tr.nwest.nhs.uk
SUMMARY
The myofibroblast is essential for the integrity of the mammalian body by virtue of its role in wound-healing, but it can also threaten it by its ability to promote tumour development. It is an almost universal cellular component in mammalian lesions, but not a typical component of normal untraumatised tissues. Partly because of its absence from normal tissue, it has not been part of conventional histology teaching. This has contributed to difficulties in appreciating the nature of the myofibroblast and defining it. This paper documents the features of the myofibroblast which provide a definition for the myofibroblast needed by scientists interested in the mechanism of disease and pathologists wanting to diagnose myofibroblastic lesions. Light microscopy features emphasised for defining the myofibroblast include: spindled cell morphology, an abundant matrix, immunostaining for a-smooth-muscle actin (in the absence of desmin and h-caldesmon) and the ED-A splice variant of cellular fibronectin. By electron microscopy, rough endoplasmic reticulum, peripherally located smooth-muscle type myofilaments, a Golgi apparatus producing collagen-secretion granules and fibronexus junctions are important. The fibronexus is emphasised as a distinctive organelle for identifying the myofibroblast and lamina is emphasised as absent. The mechanism by which myofibroblasts arise in granulation tissue and promote tumour development, and the how the above definition can be used in diagnosing myofibroblastic lesions, is discussed.
Keywords: Myofibroblast, ultrastructure, fibronexus, myofibroblastic sarcoma.
RESUMEN
El miofibroblasto es fundamental para la integridad del organismo de los mamíferos dado su papel en la curación de las heridas, pero puede resultar deletéreo por su capacidad para producir tumores. Se trata de un componente celular prácticamente universal en lesiones de mamíferos, pero no es un componente típico del tejido normal no traumatizado. Debido, en parte, a su ausencia en tejidos normales, no suele formar parte de la enseñanza convencional de la histología, lo que ha dificultado su estudio y definición. El presente trabajo documenta las características del miofibroblasto con el fin de proporcionar una definición para científicos interesados en los mecanismos de enfermedad y para histopatólogos involucrados en el diagnóstico de lesiones miofibroblásticas. Las características histológicas que permiten la identificación del miofibroblasto son: morfología fusocelular, abundante matriz y positividad inmunohistoquímica para a-actina específica de músculo liso (en ausencia de desmina y h-caldesmón) y EDA-fibronectina. En microscopía electrónica los hallazgos más importantes son: evidencia de retículo endoplásmico rugoso bien desarrollado, miofilamentos subplasmalemales de tipo muscular liso, aparato de Golgi con gránulos de secreción de colágeno y uniones tipo fibronexo que se considera como una organela característica; no debe encontrarse lámina externa. En el presente trabajo se comentan los mecanismo por los que el miofibroblasto aparece en el tejido de granulación y da lugar a tumores y como la anterior definición puede aplicarse al diagnóstico de las lesiones miofibroblásticas.
Palabras clave: Miofibroblasto, ultraestructura, fibronexo, sarcoma miofibroblástico.
INTRODUCTION
The myofibroblast is an unusual and an interesting cell for a number of reasons. It is a cellular version of the Jeckyll and Hyde character in Robert Louis Stevenson’s novel, in that it can have a benign or a malign influence depending on circumstances. By virtue of its role in wound-healing, it can promote health, but it can also endanger it by promoting the development of tumours. Understanding the biological complexity of this cell has been hindered by the fact that it has not been easy to define. First: it is not, essentially, a «normal» cell: i.e., in contrast to such well known cells as smooth-muscle cells, endothelium and pericytes, it is not found in normal untraumatised tissues. Consequently, the myofibroblast has not traditionally been featured in histology textbooks and courses for medical and science students. Second: the myofibroblast is of interest because it harbours within itself two phenotypes normally found in other cells – the fibroblast and smooth-muscle cell.
This review offers a definition of the myofibroblast, which is essential for scientists investigating the mechanism of disease in which these cells participate, and for pathologists who need to diagnose myofibroblastic lesions: it also discusses a number of aspects of myofibroblast biology which are still the subject of controversy and debate.
DEFINITION OF THE MYOFIBROBLAST: CRITERIA FOR IDENTIFICATION
It is clear from the literature that there are differing definitions of the myofibroblast, a situation partly reflecting the different scientific backgrounds of investigators (for example, whether they work in and have trained in pathology, anatomy or cell-biology) and their selective interest in the published literature.
The myofibroblast was originally defined by purely ultrastructural criteria in 1971 (1). Since then, immunohistochemistry has added its own component to the definition, while the myofibroblast also, of course, has distinctive morphological features in histological sections which cannot be divorced from the definition. So, the current definition is a complex one, reflecting the input from different techniques:
Spindle-cell or stellate-cell morphology
A pericellular matrix containing inter alia collagen and glycosaminoglycans
Palely eosinophilic and prominent cytoplasm
Immunophenotype:
Vimentin positive
a-smooth-muscle actin positive (?-SMA)
non-muscle myosin positive
minimal levels of desmin and smooth-muscle myosin
EDA cellular fibronectin positive
Ultrastructure
prominent rough endoplasmic reticulum (rER)
a Golgi apparatus producing collagen secretion granules
modestly developed and frequently peripherally located
myofilaments with focal densities
gap junctions
fibronexuses consisting of converging myofilaments and
external fibronectin fibril
absence of lamina.
This definition applies to the fully differentiated myofibroblast as found in granulation tissue or tumour stroma. But in these tissues, some cells, we presume, are evolving into a high level of myofibroblastic differentiation from more primitive precursors, and so may lack some of these features. This lesser degree of differentiation may also be seen in neoplastic myofibroblasts, where, in addition and for example, nucleoli can be expected to be enlarged, and immunophenotype expanded, reduced or aberrant (e.g., cytokeratin may be present).
DESMIN STAINING IN MYOFIBROBLASTS
In normal cells, desmin is the archetypal intermediate filament protein of muscle cells, including, of course, smooth-muscle cells: desmin is not significantly expressed in either granulation tissue or tumour stromal myofibroblasts (2,3). As such, it should not be regarded as a primary marker of the myofibroblast. However, since the study of Skalli et al (4) many authors have used desmin as a confirmatory marker for the myofibroblast. The study of Skalli et al investigated desmin in a wide range of tissues – including normal tissues such as dermis, normally healing granulation tissue, as well as lesional cells such as those in fibromatosis, and it is important to note that desmin was present only in lesional myofibroblasts.
In order to identify myofibroblastic differentiation in lesional cells, it is necessary to have a definition for what one might call the normal cellular counterpart: as mentioned above and further detailed below, myofibroblasts very largely do not exist in normal tissues, so it is necessary to identify the nearest tissue to normal, and this has been argued as the granulation tissue and tumour stromal myofibroblast. One therefore needs to be careful when assigning a cell differentiation on the basis of desmin staining in lesional cells. Strong staining will indeed suggest true smooth-muscle differentiation: lesser levels of staining will be ambiguous, and an electron microscopy input is suggested to make the distinction between smooth-muscle and myofibroblastic differentiation. In addition, if desmin staining is co-expressed with lamina, this will give stronger support to an interpretation of true smooth-muscle differentiation.
THE SPECIAL IMPORTANCE OF THE FIBRONEXUS AS A MYOFIBROBLAST MARKER
At least in the field of pathology, the fibronexus (or fibronexus junction) is less well known as a myofibroblast marker than rER and myofilaments. Partly, this is because it was first documented in the non-pathology literature (by Singer in 1979 in the journal, Cell) (5) and some time has been needed for this structure to become embedded in the pathology literature.
The fibronexus is a discrete area on the myofibroblast cell surface where the intracellular myofilaments and the extracellular fibronectin filaments (forming the fibronectin fibril) converge. The myofilaments attach to subplasmalemmal actin-binding proteins, which in turn attach to transmembrane integrins, which, on the cell exterior, attach to fibronectin. The myofilament bundle and fibronectin fibril are therefore in indirect contact and are seen, in appropriate sections, to be co-linear (5-12). The fibronexus is, therefore, a cell-to-matrix junction, ensuring a degree of adhesion to or contact with the extracellular matrix.
Much uncertainty in the field of myofibroblast biology has been generated by confusing the fibronectin fibril with lamina («external lamina»), one of the few other structures associated with the external surface of cells. The fibronectin fibril and lamina are quite different structures, with different functions and different significances for cell differentiation.
The fibronectin fibril has the following features, which distinguish it from lamina (figs. 1-3):
it is denser and straighter than lamina
it has a longitudinal and finely filamentous substructure, which is lacking in lamina
it projects (usually at a small angle) from the cell surface into the extracellular space, whereas lamina usually follows the contours of the cell with which it is associated
it is co-linear with intracellular myofilaments
it is often seen «attaching» to the cell surface at a localised cell surface inclination.

Fig. 1: A
myofibroblast and myofibroblast processes in squamous cell carcinoma stroma. rER
and peripheral myofilaments are present, and dense fibrillar fibronectin is
evident at cell surfaces.

Fig. 2: Detail
of myofilaments and fibronectin fibrils forming a fibronexus. The fibronectin
projects out into the matrix.
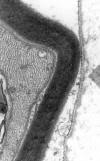
Fig. 3: Lamina
over the surface of a Schwann cell: note how it follows the contour of the cell
surface, in contrast to the fibronectin in figures 1 and 2.
Immuno-electronmicroscopy has confirmed the fibronectin content of the fibronectin fibril (8,10,13). The structures immuno-ultrastructurally labelled by anti-fibronectin antibodies in the study by Tamm et al (14) are also probably related to fibronectin fibrils. It is likely that the fibronectin at the fibronexus contains the myofibroblast-specific ED-A isoform (15,16). While lamina also contains some fibronectin, it is rich in proteins such as laminin, type IV collagen and proteoglycans (17).
On the specificity of the fibronexus, the published literature points to it as a highly characteristic marker organelle of the myofibroblast (18-21). However, like nearly all markers, whether immunohistochemical or ultrastructural, it is not completely specific. Rare examples of endothelium exhibit fibronexuses (22,23), mostly in the aorta, possibly as an adaptation to haemodynamic stress. In attenuated form, they have been noted in certain vascular smooth-muscle cells (9,24), whereas in bovine arteriosclerosis, structures resembling fibronectin fibrils appear to be prominent (25). In addition, normal cells, which in the in vivo state are non-myofibroblastic, can assume myofibroblastic features including formation of the fibronexus when cultured in vitro: examples include fibroblasts, smooth muscle cells and epithelium (5,26). This represents transdifferentiation towards the myofibroblast phenotype of an initially non-myofibroblastic cell.
ABSENCE OF THE MYOFIBROBLAST FROM NORMAL TISSUES
The myofibroblast is archetypally found in granulation tissue, non-neoplastic fibrosing or fibro-contractive conditions, and tumour stroma (18,19,27-32). Like nearly all cells, in addition, it can be neoplastic (see below). Apart from the tumoral counterpart, the myofibroblast is essentially a reactive cell, and by that is meant a cell appearing in conditions generated by externally applied trauma or inherent abnormality, as in tumour stroma.
This essentially reactive nature of the non-neoplastic myofibroblast conflicts with a number of references in the literature describing the myofibroblast as being found in normal organs and tissues (27,28,33,34). It is probably true that all tissues harbour mesenchymal or fibroblastic cells which have the potential to become activated to a myofibroblast as a result of externally applied trauma, but to say that all tissues have myofibroblasts implies that all tissues are traumatised, which is clearly not the case. It is more reasonable, perhaps, to take the view that some stromal cells showing very minor degrees of myofibroblastic differentiation resulting from comparably minor and possibly transient states of trauma or stress. Consequently, most stromal cells in normal tissues do not show significant myofibroblastic differentiation, and those that do, show it only to a very minor degree. The periodontal myofibroblast and the interstitial cells of the mammalian testis are two exceptions.
BIOLOGICAL FUNCTIONS OF THE MYOFIBROBLAST
Wound-healing
This process, by which physical integrity of mammalian tissues is ensured after injury, consists of several steps. One of the earliest is the transdifferentiation of surrounding resident stromal cells, fibroblasts as often as not, into myofibroblasts. This process involves the switching on of non-muscle actin in cells having a spindled or flattened morphology, rER, and stress fibres lacking a-SMA: they have been referred to as protomyofibroblasts (35,36). The protomyofibroblast is, therefore, one of the earliest phases in the transition of a fibroblast to a myofibroblast.
Several studies have indicated that the entire process leading to the complete myofibroblast phenotype requires the concerted action of growth factors (such as transforming growth factor b, [TGFb]), matrix molecules, and a mechanically stressed environment (15,16,35-38). Two factors appear to be important in the earliest stages of the development of the myofibroblast: platelet-derived growth factor (PDGF) released from the blood in a wound acting as a mitogen or chemo-attractant for resident fibroblasts (39); and PDGF interacting with these PDGF-receptor bearing cells and, with the involvement of other cytokines, precipitating them into a differentiation process which ultimately leads to the myofibroblast.
As already mentioned, another early signal for protomyofibroblast formation is mechanical tension. The effect of mechanical stress in the formation of stress fibres (bundles of actin filaments) attached to membranes was demonstrated by Brandes et al (40) (see also: 15,16,41). Endothelial cells in stressed tissues showed enhanced actin filament bundles compared with non-stressed specimens. It is arguable that the appearance of a-SMA in cardiac fibroblasts results from ventricular pressure overload (42) and is an example of stress-related myofibroblastic development. The stressing would appear to be a reactive mechanism by which cell and matrix cohesion is ensured, in much the same way, perhaps, that aortic endothelial cells elaborate fibronexus junctions with the extracellular matrix under conditions of high haemodynamic stress (22).
Before inducing a-SMA, however, TGFb induces the synthesis of fibronectin, and collectively TGFb and fibronectin direct a-SMA synthesis. TGFb regulates the levels and isoform patterns of fibronectin (43), especially the ED-A and ED-B splice-variants (44), which then exert a «permissive» action for the expression of a-SMA (35,39,45). The ED-A variant of fibronectin, in particular, can be regarded as important a marker for the myofibroblast as a-SMA.
Myofibroblasts and tumour promotion
There is growing evidence that myofibroblasts promote tumour development, and act in concert with neoplastic cells (46-53). This conflicts with the early idea that abundant matrix synthesized by the myofibroblast formed a physical barrier inhibiting tumour cell movement and amounted to a protective measure for the host (54-56).
The tumour-promoting effect is probably based on the direct cytokine-stimulation of cancer cells, the maintenance of vascularity, but also partly on the capacity of myofibroblasts to produce enzymes which degrade either matrix (50) or molecules which enhance the structural integrity of matrix, such as lysyl oxidase (this promotes collagen and elastin crosslinking, and is decreased in invasive compared with in situ carcinomas – 57). Matrix-degrading enzymes include metalloproteinases (58,59): in principle, such activity would create easier physical access for neoplastic cells to the vasculature –an early step in the metastatic process– but also it seems likely that it would produce new molecules with enhanced activity with regard, for example, to the migratory activity needed to access vessels (59).
Another currently prevalent idea is that myofibroblasts create a physical barrier between carcinoma cells, on the one hand, and the macrophages and T cells, on the other, which are part of the system attempting to mount an immune defence on the part of the body again the cancer (50,52). The many images in the published literature of myofibroblasts in close association with carcinoma cells would be consistent with this idea (28,60).
Mechano-reception and the detection of stress
The fibronexus is regarded as a transmembrane cell-to-matrix adhesive or junctional device. While its functions are not unambiguously clear as yet, it may have a role in transferring the intracellular contractility through the cell surface to the matrix, in such processes as wound-contraction (35,61). At the same time, it has been recognised that fibroblasts, not having differentiated into myofibroblasts, can contract tissue matrices, presumably by tractional forces (62). More recently, the idea has been proposed that fibronexuses may detect tension in the extracellular matrix (15,16,35,63) and thereby act as mechano-transducers, converting the energy of external mechanical stress into biological activity (cell-signalling, de novo protein synthesis and new phenotypes). This is an exciting area of investigation where physics and biology interface.
ORIGIN AND FATE
Myofibroblasts have traditionally been argued as deriving mainly from locally resident fibroblasts (62,64,65) but also smooth-muscle cells, pericytes (3,52,66), macrophages (67,68), as well as other «more specialised» cells such as hepatic stellate cells (66) and epithelium (69). Ultimately, they may derive from bone marrow via circulating blood-borne fibrocytes (70-74).
Towards the conclusion of wound-healing, myofibroblasts, along with cells of the neovasculature, disappear by apoptosis (66,75). When the apoptotic mechanism fails, prolonged scarring results, leading to such conditions as hypertrophic scar and keloid.
MYOFIBROBLASTIC DIFFERENTIATION IN TUMOURS AND TUMOUR-LIKE LESIONS
Using the definition detailed above, it is clear that a spectrum of myofibroblastic differentiation exists in the tumours and tumour-like lesions that are often referred to as fibroblastic/myofibroblastic. The lesions containing the most highly differentiated myofibroblasts include nodular and proliferative fasciitis, Dupuytren’s disease and the other (myo)fibromatoses, inflammatory myofibroblastic tumour, and some myofibroblastic sarcomas (76).
There are good grounds based on ultrastructure and desmin immunostaining for regarding some of the lesions widely regarded and referred to as myofibroblastic as showing, rather, a low level of true smooth-muscle differentiation. While many of the ultrastructurally examined fibromatoses exhibit fibronexus junctions and so are fully myofibroblastic, a few others have the lamina indicative of smooth-muscle cells (77) or are strongly desmin-positive (78). By contrast, nodular fasciitis is uniformly negative for desmin (79,80), further emphasising its «true» or «complete» myofibroblastic phenotype.
The features of attachment plaques, caveolae and lamina have been seen in a number of myofibroblastomas (81-85) and angiomyofibroblastoma (86), which, in the absence of fibronexus junctions, suggest true smooth-muscle differentiation.
In poorly differentiatiated spindle cell tumours, the distinction between myofibroblastic differentiation and, for example, leiomyosarcomatous differentiation becomes difficult, particularly, when a cell has rER and peripheral myofilaments but no identifiable fibronexuses. This raises the question: does one need the fibronexus for the identification of myofibroblastic differentiation in a spindle-cell sarcoma? Although in earlier papers the fibronexus was indeed emphasised as an essential part of the definition of the myofibroblast (9), it might now be more reasonable to think in terms of levels of differentiation and levels of diagnostic confidence. If, in a spindle-cell sarcoma, there are features of myofibroblastic differentiation (delicate a-smooth-muscle actin, rER, myofilaments, no lamina, no desmin, no h-caldesmon) but no fibronexuses, it may still be reasonable to call this a myofibroblastic sarcoma (a poorly differentiated one) in the absence of any other compelling diagnosis. However, maximum diagnostic confidence of a myofibroblastic tumour would be achieved by identifying fibronexuses, which in turn requires electron microscopy.
ACKNOWLEDGEMENT
Several parts of this review derive from my two papers published in the Journal of submicroscopic Cytology and Pathology in 2005 (76,87). I am most grateful to the Managing Editor, Laura Neri, for kind permission to make use of the text and figures in these papers for this review.
REFERENCES
Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971; 27: 549-550.
Truong LD, Rangdaeng S, Cagle P, Ro JY, Hawkins H, Font RL. The diagnostic utility of desmin. A study of 584 cases and review of the literature. Am J Clin Pathol 1990; 93: 305-314.
Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995; 95: 859-873.
Skalli O, Schürch W, Seemayer T, Lagacé R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 1989; 60: 275-285.
Singer II. The fibronexus. A transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 1979; 16: 675-685.
Bjørkerud S, Gustavsson K, Hasselgren M. In vitro cultivation of rabbit aortic media and the development of the cultures in relation to cellular heterogeneity. Acta Path Microbiol Immunol Scand Sect A 1984; 92: 113-124.
Singer II. Fibronectin-cytoskeleton relationships. In: Fibronectin, Mosher D.F., ed., Academic Press, San Diego, 1989; 139-161.
Singer II, Kawka DW, Kazazis DM, Clark RAF. In vivo codistribution of fibronectin and actin fibers in granulation tissue. Immunofluorescence and electron microscope studies at the myofibroblast surface. J Cell Biol 1984; 98: 2091-2106.
Eyden BP. Brief review of the fibronexus and its significance for myofibroblastic differentiation and tumor diagnosis. Ultrastruct Pathol 1993; 17: 611-622.
Eyden BP. The fibronexus in reactive and tumoral myofibroblasts: further characterisation by electron microscopy. Histol. Histopathol 2001; 16: 57-70.
Eyden BP. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol 2001; 25: 39-50.
Eddy RJ, Petro JA, Tomasek JJ. Evidence for the nonmuscle nature of the «myofibroblast» of granulation tissue and hypertrophic scar. An immunofluorescence study. Am J Pathol 1988; 130: 252-260.
Singer II, Kazazis DM, Kawka DW. Localization of the fibronexus at the surface of granulation tissue myofibroblasts using double-label immunogold electron microscopy on ultrathin frozen sections. Eur J Cell Biol 1985; 38: 94-101.
Tamm E, Baur A, Lütjen-Drecoll E. Synthesis of extracellular matrix components by human ciliary muscle cells in culture. Curr Eye Res 1992; 11: 333-341.
Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 2003; 14: 538-546.
Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodelling. Thromb Haemost 2003; 90: 993-1002.
Abraham DR. Recent studies on the structure and pathology of basement membranes. J Pathol 1986; 149: 257-278.
Tremblay G. Stromal aspects of breast carcinoma. Exp Mol Pathol 1979; 31: 248-260.
Seemayer TA, Lagacé R, Schürch W, Thelmo WL. The myofibroblast: biologic, pathologic, and theoretical considerations. Pathol Annu 1980; 15: 443-470.
Baur PS JR, Parks DH. The myofibroblast anchoring strand - the fibronectin connection in wound healing and the possible loci of collagen fibril assembly. J Trauma 1983; 23: 853-862.
Schürch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery [editorial]. Am J Surg Pathol 1998; 22: 141-147.
Hüttner I, Walker C, Gabbiani G. Aortic endothelial cell during regeneration. Remodeling of cell junctions, stress fibers, and stress fiber-membrane attachment domains. Lab Invest 1985; 53: 287-302.
Skalli O, Gabbiani G. The biology of the myofibroblast. Relationship to wound contraction and fibrocontractive diseases. In: The molecular and cellular biology of wound repair, CLARK R.A.F. and HENSON P.M., eds, Plenum Press, New York, 1988; 373-402.
Dingemans KP, Teeling P, Lagenduijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec 2000; 258: 1-14.
Knieriem HJ. Electron-microscopic study of bovine arteriosclerotic lesions. Am J Pathol 1976; 50: 1035-1065.
Toselli P, Faris B, Oliver P, Franzblau C. Ultrastructural studies of attachment site formation in aortic smooth muscle cells cultured on collagen-hydroxyethylmethacrylate hydrogels. J Ultrastruct Res 1984; 86: 252-261.
Guber S, Rudolph R. The myofibroblast. Surgery Gynecol Obstet 1978; 146: 641-649.
Lipper S, Kahan LB, Reddick RL. The myofibroblast. Pathol Annu 1980; 15: 409-441.
Tamimi SO, Ahmed A. Stromal changes in invasive breast carcinoma: an ultrastructural study. J Pathol 1987; 153: 163-170.
Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 1990; 63: 144-161.
Schürch W, Skalli O, Gabbiani G. Cellular Biology. The myofibroblast. Definition, ultrastructural features and role in wound contraction (Chapter 4). In: Dupuytren’s disease. Biology and treatment, McFarlane RM, MCGrouther DA and Flint MH, eds, Churchill Livingstone, Edinburgh, 1990; 31-47.
Schmitt-Gräff A, Desmoulière A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblast cell plasticity. Virchows Arch 1994; 425: 3-24.
Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Path Res Pract 1996; 192: 712-717.
Mentzel T, Dry S, Katenkamp D, Fletcher CDM. Low-grade myofibroblastic sarcoma. Analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol 1998; 22: 1228-1238.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Rev Mol Cell Biol 2002; 3: 349-363.
Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003; 200: 500-503.
Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-b1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002; 39: 258-263.
Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. a-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 2003; 14: 2508-2519.
Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999; 250: 273-283.
Brandes G, Reale E, Messina A. Microfilament system in the microvascular endothelium of the palmar fascia affected by mechanical stress applied from outside. Virchows Arch 1996; 429: 165-172.
Hinz B, Mastrangelo D, Iselion CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 2001; 159: 1009-1020.
Leslie KO, Taatjes DJ, Schwartz J, Von Turkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol 1991; 139: 207-216.
Borsi L, Castellani P, Risso AM, Leprini A, Zardi L. Transforming growth factor-beta regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Lett 1990; 261: 175-178.
Berndt A, Borsi L, Luo X, Zardi L, Katenkamp D, Kosmehl H. Evidence of ED-B+ fibronectin synthesis in human tissues by non-radioactive RNA in situ hybridization. Investigations on carcinoma (oral squamous cell and breast carcinoma), chronic inflammation (rheumatoid arthritis) and fibromatosis (Morbus Dupuytren). Histochem Cell Biol 1998; 109: 249-255.
Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci 2001; 114: 3285-3296.
Hernandez AD, Hibbs MS, Postlethwaite AE. Establishment of basal cell carcinoma in culture: evidence for a basal cell carcinoma-derived factor(s) which stimulates fibroblasts to proliferate and release collagenase. J Invest Dermatol 1985; 85: 470-475.
Lipponen P, Aaltomaa S, Papinaho S, Syrjänen K. Nucleolar organizer regions in myofibroblasts in breast cancer. Relation to cancer cell morphometry, flow cytometry, sex steroid receptor content, tumour histology and prognosis. Path Res Pract 1993; 189: 1030-1035.
Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol 2002; 3: 35-43.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002; 8: 2912-2923.
De Wewer O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol 2003; 200: 429-447.
Chung LWK. The roles of myofibroblasts in prostate carcinogenesis. J Urol 2004; 172: 2125-2126.
Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behaviour. Int J Dev Biol 2004; 48: 509-517.
Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous cell carcinoma cells. Br J Cancer 2004; 90: 822-832.
Lagacé R, Schürch W, Seemayer TA. Myofibroblasts in soft tissue sarcomas. Virchows Arch A Path Anat Histol 1980; 389: 1-11.
Seemayer TA, Schürch W, Lagacé R. The myofibroblast and defense against neoplasia: a hypothesis. Surv Immunol Res 1982; 1: 268-273.
Sano T, Ueki M. Stromal reactions to squamous cell carcinoma of the cervix. Am J Obstet Gynecol 1987; 156: 906-910.
Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol 1997; 150: 497-507.
Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol 1996; 3: 895-904.
Stamenkovich I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 2003; 200: 448-464.
Ahmed A. The myofibroblast in breast disease. Pathol Annu 1990; 25: 237-286.
Garana RMR, Petroll WM, Chen W-T, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci 1992; 33: 3271-3282.
Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994; 124: 401-404.
Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol 2006; 85: 175-181.
Bouissou H, Pieraggi M, Julian M, Uhart D, Kokolo J. Fibroblasts in dermal tissue repair. Electron microscopic and immunohistochemical study. Int J Dermatol 1988; 27: 564-570.
Faggian L, Pampinella F, Roelofs M, Paulon T, Franch R, Chiavegato A, Sartore S. Phenotypic changes in the regenerating rabbit bladder muscle. Role of interstitial cells and innervation on smooth muscle cell differentiation. Histochem Cell Biol 1998; 109: 25-39.
Gabbiani G. The cellular derivation and the life span of the myofibroblast. Path Res Pract 1996; 192: 708-711.
Bhawan J, Majno G. The Myofibroblast. Possible derivation from macrophages in xanthogranuloma. Am J Dermatopathol 1989; 11: 255-258.
Bayes-Genis A, Campbell JH, Carlson PJ, Holmes DR, Schwartz RS. Macrophages, myofibroblasts and neointimal hyperplasia after coronary artery injury and repair. Atherosclerosis 2002; 163: 89-98.
Tian Y-C, Fraser D, Attisano L, Phillips AO. TGF-b1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol Renal Physiol 2003; 285: F130-F142.
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001; 166: 7556-7562.
Schmidt TM, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003; 170: 380-389.
Quan TE, Cowper C, Wu S-P, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004; 36: 598-606.
Yamaguchi Y, Kubo T, Murakami T, Takahashi M, Hakamata Y, Kobayashi E, Yoshida S, Hosokawa K, Yoshikawa K, Itami S. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br J Dermatol 2005; 152: 616-622.
Jabs A, Moncada GA, Nichols CE, Waller EK, Wilcox JN. Peripheral blood mononuclear cells acquire myofibroblastic characteristics in granulation tissue. J Vasc Res 2005; 42: 174-180.
Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995; 146: 56-66.
Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 2 – Tumours and tumour-like lesions. J Submicrosc Cytol Pathol 2005; 37: 231-296.
Takagi M, Yamamoto H, Mega H, Hsieh KJ, Shioda S, Enomoto S. Heterogeneity in the gingival fibromatoses. Cancer 1991; 68: 2202-2212.
Hasegawa T, Hirose T, Kudo E, Abe J-I, Hizawa K. Cytoskeletal characteristics of myofibroblasts in benign neoplastic and reactive fibroblastic lesions. Virchows Arch A Pathol Anat 1990; 416: 375-382.
Montgomery EA, Meis JM. Nodular fasciitis. Its morphologic spectrum and immunohistochemical profile. Am J Surg Pathol 1991; 15: 942-948.
Thompson LDR, Fanburg-Smith JC, Wenig BM. Nodular fasciitis of the external ear region: a clinicopathologic study of 50 cases. Ann Diagn Pathol 2001; 5: 191-198.
Eyden BP, Harris M, Greywoode GIN, Christensen L, Banerjee SS. Intranodal myofibroblastoma: report of a case. Ultrastruct Pathol 1996; 20: 79-88.
Eyden BP, Shanks JH, Ioachim E, Ali HH, Christensen L, Howatt AJ. Myofibroblastoma of breast: evidence favoring smooth-muscle rather than myofibroblastic differentiation. Ultrastruct Pathol 1999; 23: 249-257.
Eyden B, Chorneyko K. Intranodal myofibroblastoma: study of a case suggesting smooth-muscle differentiation. J Submicrosc Cytol Pathol 2001; 33: 157-163.
Michal M, Chlumská A, Skálová A, Fakan F. Palisaded intranodal myofibroblastoma. Electron microscopic study. Zentralbl F Pathol 1993; 139: 81-88.
Tanda F, Massarelli G, Cossu A, Bosincu L, Cossu S, Ibba M. Primary spindle cell tumor of lymph node with «amianthoid» fibers: a histological, immunohistochemical and ultrastructural study. Ultrastruct Pathol 1993; 17: 195-205.
Fletcher CDM, Tsang WYW, Fisher C, Lee KC, Chan JKC. Angiomyofibroblastoma of the vulva. A benign neoplasm distinct from aggressive angiomyxoma. Am J Surg Pathol 1992; 16: 373-382.
Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 1 – normal and reactive cells. J Submicrosc Cytol Pathol 2005; 37: 109-204.